Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Descriptive Statements:
- Analyze atomic systems using experimental evidence, the quantum mechanical model, and the development of historical models (e.g., Dalton's, Thomson's, Rutherford's).
- Demonstrate knowledge of the comparative characteristics of subatomic particles (i.e., mass, charge, and location within an atom).
- Demonstrate knowledge of the relationship between atomic number, neutron-to-proton ratio, and nuclear stability within an atom.
- Demonstrate knowledge of the relationship between an atom's mass number and the element's atomic mass and isotopic masses.
- Apply knowledge of the formation of new elements through nuclear processes (e.g., fission, fusion, alpha or beta decay).
- Demonstrate knowledge of electron configurations under various models and representations (e.g., Bohr diagram, subshell list, Lewis dot diagram, orbital diagram).
- Apply knowledge of the electromagnetic spectrum and wave-particle duality to explain the electronic characteristics of atomic systems.
Sample Item:
Which of the following events only occurs during a nuclear change?
- Valence electrons are raised to higher energy levels.
- Two or more types of atoms form a compound.
- Energy is released to the surroundings.
- An element's atomic number is reduced.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
D. Chemical and physical changes do not involve changes within the nucleus of an atom and therefore would not lead to a reduction in an element's atomic number. This type of change would only result from a nuclear change.
Descriptive Statements:
- Demonstrate knowledge of physical and chemical properties and their role in the identification and categorization of elements.
- Apply knowledge of the structure of the periodic table and its dependence on atomic structure (e.g., periods, groups, lanthanides, metals, nonmetals).
- Analyze data on the periodic trends in the properties of elements (e.g., atomic radius, electron affinity, electronegativity, ionization energy).
- Apply knowledge of atomic structure, Coulomb's law, and quantum mechanics to explain trends in the properties of elements.
Sample Item:
Which of the following elements is the most electronegative?
- hydrogen (H)
- fluorine (F)
- radon (Rn)
- francium (Fr)
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
B. The electronegativity of an element scales with its attraction for an additional electron and tends to increase from bottom to top within a group and from left to right across a period on the periodic table. Of the given elements, fluorine is in the uppermost position on the right-hand side of the periodic table.
Descriptive Statements:
- Demonstrate knowledge of various types of chemical bonds (e.g., ionic, covalent, metallic) and their characteristics.
- Apply knowledge of atomic structure and bonding characteristics to explain trends in the properties of compounds.
- Apply knowledge of atomic structure and electrostatics to the formation of chemical bonds, including predictions of an element's bonding behavior (e.g., constituent atoms, bond energies, covalent bond length, ionic internuclear distance).
- Apply knowledge of simple bonding models (e.g., Lewis structures, electron density diagrams, structural formulas) to explain the formation of compounds.
- Apply knowledge of the valence-shell-electron-pair repulsion (VSEPR) theory connected to predictions of molecular geometry (i.e., shape, angle, and structure name) and polarity.
- Analyze the structures and characteristics of large organic molecules using knowledge of bonding characteristics, polarity, and intermolecular forces.
- Demonstrate knowledge of chemical formulas and names for inorganic and simple organic compounds according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature guidelines.
Sample Item:
Which of the following statements explains why nitric acid (HNO3 H N O 3 ) is a stronger acid than nitrous acid (HNO2 H N O 2 )?
- The additional oxygen present in nitric acid increases the polarity of the O—H O H bond.
- The extent of ionization is directly related to molecular weight when comparing related compounds.
- The anion formed by removing H+ H positive from nitrous acid is more stable than the anion formed by removing H+ H positive from nitric acid.
- The O—H O H bond in nitrous acid is weaker than the O—H O H bond in nitric acid.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
A. The strength of an acid is a function of its tendency to ionize. For oxoacids with the same central atom, acid strength increases as the oxidation number of the central atom increases because of the resulting increase in polarity of the O—H O H bond. The oxidation number of nitrogen in HNO3 H N O 3 is +5 plus 5 and in HNO2 H N O 2 it is +3 plus 3 , thus the O—H O H bond in HNO3 H N O 3 is more polar and ionizes more readily.
Descriptive Statements:
- Demonstrate knowledge of the physical properties, chemical properties, and characteristics of mixtures (e.g., solutions, homogeneous, heterogeneous).
- Demonstrate knowledge of how a mixture's particle size and composition affect its properties (e.g., colligative properties of a solution, density of an alloy, Tyndall effect in colloids).
- Apply knowledge of the separation of mixtures based on their physical and chemical properties.
- Apply knowledge of concentration units and calculations using concentration (e.g., molarity, parts per million, pH).
- Demonstrate knowledge of factors affecting solubility (e.g., temperature, pressure, strength of particle interactions).
- Demonstrate knowledge of the structures and characteristics of acids and bases.
Sample Item:
Use the graph below to answer the question that follows.
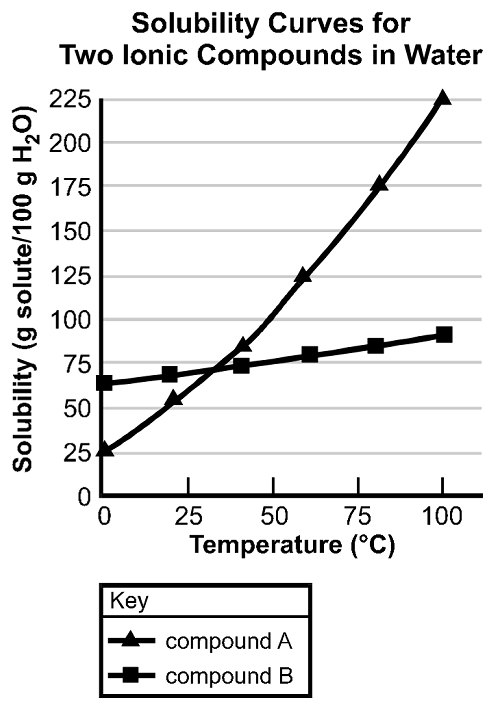
The y axis is labeled Solubility and is measured in units of grams of solute per one hundred grams of water. The scale ranges from zero to two hundred fifty-five. The x axis is labeled Temperature and is measured in degrees Celsius. The scale ranges from zero to one hundred. Two lines are shown on the chart, one for compound A and one for compound B. The compound A line passes through the points x equals zero y equals twenty-five, x equals twenty-five y equals fifty-five, x equals forty-five y equals eighty-five, x equals sixty y equals one hundred twenty-five, x equals eighty y equals one hundred seventy-five, and x equals one hundred y equals two hundred twenty-five. The compound B line passes through the points x equals zero y equals sixty-five, x equals twenty y equals seventy, x equals forty y equals seventy-five, x equals sixty-five y equals eighty, x equals eighty y equals eighty-five, x equals one hundred y equals ninety.
Based on the solubility curves shown, which of the following procedures will be most effective in isolating the greatest amount of pure compound A from a mixture consisting of 200 g grams of compound A and 15 g grams of compound B?
- dissolving the mixture in 100 g grams of water and then heating to the solution's boiling point
- dissolving the mixture in 100 g grams of water at 100°C degrees Celsius and then decreasing the temperature to 0°C degrees Celsius
- dissolving the mixture in 100 g grams of water at 75°C degrees Celsius , filtering the solution, and then retaining the filtrate
- dissolving the mixture in 100 g grams of water and then slowly increasing the temperature to 100°C degrees Celsius
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
B. Based on the solubility curves provided, both compound A and compound B will be completely in solution when dissolved in 100 g grams of water at 100°C degrees Celsius . As the temperature of the solution is decreased to 0°C degrees Celsius , 175 g grams of compound A will precipitate out of the solution, while all of compound B will remain dissolved. This is the procedure that yields the most compound A.
Descriptive Statements:
- Demonstrate knowledge of various types of intermolecular forces.
- Apply knowledge of atomic structure, electrostatic forces, and chemical bonding to compare the strengths of intermolecular forces in various substances.
- Demonstrate knowledge of the relative spacing, motion, and energy of particles in various states of matter (e.g., solids, liquids, gases).
- Apply knowledge of how intermolecular forces affect properties (e.g., vapor pressure, melting point, boiling point) and phase changes of pure substances.
- Demonstrate knowledge of the characteristics of various types of solids (i.e., ionic, molecular, network, and metallic).
- Apply knowledge of the kinetic molecular theory of gases to predict qualitative changes in gas systems.
- Analyze the behavior of gases using mathematical relationships between bulk properties (e.g., the ideal gas law, Dalton's law of partial pressures, van der Waals equation for real gases).
Sample Item:
A gas occupies a volume of 1.25 liters at a pressure of 825 mm Hg millimeters H G . What will be the final pressure of this gas if it is compressed into a volume of 725 mL milliliters at constant temperature?
- 479 mm Hg millimeters H G
- 748 mm Hg millimeters H G
- 1.30 × 1031.30 times 10 cubed mm Hg millimeters H G
- 1.42 × 1031.42 times 10 cubed mm Hg millimeters H G
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
D. If the temperature and number of moles of a gas are held constant, the relationship between an initial pressure and volume and a new pressure and volume is P1V1 = P2V2P subscript 1 V subscript 1 equals P subscript 2 V subscript 2. This relationship can be used to calculate the new pressure when the initial pressure, initial volume, and new volume are known.

